Abstract
This study aimed to elucidate the role of peroxisome proliferator-activated receptor-γ (PPAR-γ) in regulating macrophage efferocytosis during the pathogenesis of chronic apical periodontitis (CAP). Clinical specimens, rat periapical lesion models, and an in vitro model simulating the CAP inflammatory milieu were employed to examine the contribution of PPAR-γ to efferocytosis throughout disease progression. The expression of PPAR-γ in vivo was assessed by single-cell RNA sequencing and immunohistochemical (IHC) staining. Pearson’s correlation and linear trend tests were conducted to investigate the association between PPAR-γ and macrophage efferocytosis during CAP progression. Pharmacological modulation of PPAR-γ was further conducted using rosiglitazone (RSG) as an agonist and GW9662 as an antagonist, followed by an assessment of efferocytosis-related parameters and inflammatory responses. Both clinical specimens and animal models demonstrated a progressive reduction in PPAR-γ expression and macrophage efferocytosis during CAP. Notably, PPAR-γ attenuated efferocytosis impairment and significantly reduced pathogen-induced inflammatory responses in macrophages. These findings indicate that defective macrophage efferocytosis contributes to the exacerbation of CAP severity, whereas targeting PPAR-γ may represent a promising therapeutic strategy to alleviate inflammation in periapical lesions by restoring efferocytic capacity. Collectively, this study highlights PPAR-γ as a potential therapeutic target warranting further investigation in CAP treatment.
Keywords:
apical periodontitis; PPAR-γ; macrophage; efferocytosis
1. Introduction
Chronic apical periodontitis (CAP) is a prevalent and progressive oral pathology characterized by the resorption of periapical alveolar bone (Figure 1) [1,2]. Epidemiological studies showed that about 52% of the global adult population has at least one tooth with periapical periodontitis [3]. This chronic inflammatory condition arises from a complex interaction among microbial agents, inflammatory mediators, and immune responses, ultimately resulting in alveolar bone loss and tooth retention challenges, with potential systemic implications [4,5]. Conventionally, root canal treatment (RCT), which involves thorough debridement followed by hermetic sealing of infected root canal spaces, is the primary treatment modality aimed at eradicating intracanal infection and avoiding reinfection. However, it is important to recognize that approximately 4% to 15% of treated teeth may experience persistent pain necessitating extraction post-RCT [6,7]. Furthermore, accumulating evidence has linked CAP with systemic conditions such as cardiovascular disease, rheumatoid arthritis, depression, and anxiety, underscoring the urgent need to develop novel and effective therapeutic strategies for CAP [8].
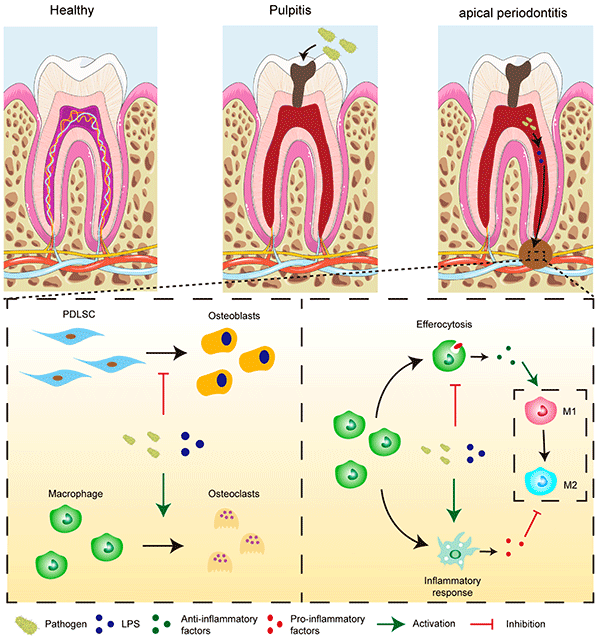 Figure 1. Pathophysiology of apical periodontitis.
Figure 1. Pathophysiology of apical periodontitis.
Macrophages, as essential components of the innate immune system and the primary defense against microbial invasion, play a vital role in the progression of CAP [9]. Growing evidence underscores the importance of macrophage efferocytosis—the process by which macrophages, known as efferocytes, remove apoptotic neutrophils from inflamed tissues, thereby influencing their polarization state [10,11]. Upon engulfment of apoptotic cells, efferocytes secrete a spectrum of bioactive mediators and extracellular vesicles that can be absorbed by surrounding macrophages, promoting a phenotypic transition toward the anti-inflammatory M2 subtype [12]. Our earlier work revealed that macrophage efferocytosis is progressively impaired as CAP advances, as evidenced in clinical samples [13]. This reduction in efferocytic activity was linked to increased M1 polarization and a higher M1/M2 ratio. Conversely, enhancing efferocytosis facilitated M1-to-M2 conversion and alleviated disease progression [13]. Collectively, these findings indicate that targeting macrophage efferocytosis may represent a promising therapeutic avenue for CAP management (Figure 2).
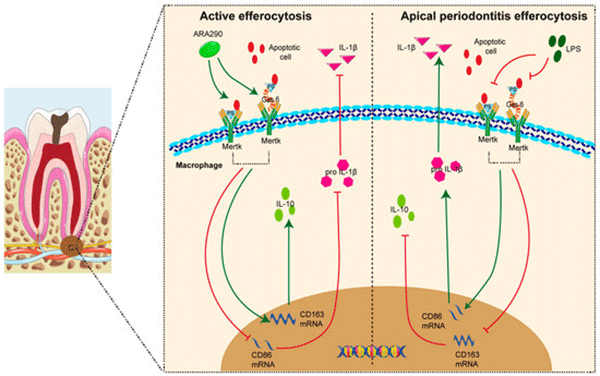 Figure 2. Mechanisms and molecular implications of macrophage efferocytosis in CAP.
Figure 2. Mechanisms and molecular implications of macrophage efferocytosis in CAP.
The article is reprinted from MDPI, original link:https://www.mdpi.com/1422-0067/26/20/10157
The FAI climbed 5.9 percent year-on-year in the first 11 months of 2018, quickening from the 5.7-percent growth in Jan-Oct, the National Bureau of Statistics (NBS) said Friday in an online statement.
The key indicator of investment, dubbed a major growth driver, hit the bottom in August and has since started to rebound steadily.
In the face of emerging economic challenges home and abroad, China has stepped up efforts to stabilize investment, in particular rolling out measures to motivate private investors and channel funds into infrastructure.
Friday's data showed private investment, accounting for more than 60 percent of the total FAI, expanded by a brisk 8.7 percent.
NBS spokesperson Mao Shengyong said funds into weak economic links registered rapid increases as investment in environmental protection and agriculture jumped 42 percent and 12.5 percent respectively, much faster than the average.
In breakdown, investment in high-tech and equipment manufacturing remained vigorous with 16.1-percent and 11.6-percent increases respectively in the first 11 months. Infrastructure investment gained 3.7 percent, staying flat. Investment in property development rose 9.7 percent, also unchanged.
 English
English