CAR-T therapy is one of the current popular tumor treatment methods. Through the modification of T cells, immune cells can accurately attack cancer cells without harming normal cells, creating a new era of tumor treatment. Since Fosun Kate Aquilunsa Injection was approved for marketing as the first domestic CAR-T cell therapy product in June last year, domestic pharmaceutical companies have been enthusiastic about research on CAR-T products. Since 2022, a number of CAR-T products in China have successively entered the NDA or Pre-NDA stage.
1. IASO Biotherapeutics/Innovent Biologics
On August 19, 2022, the official website of the Center for Drug Evaluation (CDE) of the State Food and Drug Administration of China announced that the BCMA-targeted CAR-T product Yijirenzai Injection, jointly developed by IASO Biotherapeutics and Innovent Biologics, obtained two new clinical trials. Trial implied permission, intended for the treatment of AQP4-IgG positive neuromyelitis optica spectrum disease.
On November 14, 2022, Signal Transduction and Targeted Therapy, a sub-journal of Nature, officially received the mid-term results of the IIT clinical trial of IASO Biotherapeutics BCMA CAR-T CT103A (Ikilunsai injection) in the treatment of neuromyelitis optica spectrum disorders (NMOSD), Preliminary demonstration of good tolerability and safety, durable pathogenic antibody clearance and potential clinical efficacy of BCMA CAR-T therapy in NMOSD, providing a new treatment for antibody-mediated autoimmune diseases train of thought.
2. CARsgen
On October 18, 2022, CARsgen Innovative CAR-T Cell Therapy Company announced that the National Medical Products Administration (NMPA) of China has accepted the new drug of Zevorcabtagene autoleucel injection (R&D code: CT053) The listing application (NDA) was subsequently placed for Priority Review on October 21. Zewoki Orensa injection is a fully human anti-autologous BCMA CAR-T cell product candidate for the treatment of relapsed/refractory multiple myeloma.
3. Juventas
On October 11, 2022, Juventas and the Hematology Hospital of the Chinese Academy of Medical Sciences jointly announced that the CD19-targeting CAR-T product Hejilunsai Injection (proposed) is effective in the treatment of adult relapsed or refractory acute B-lymphoblastic leukemia (r/r B-ALL) reached the main endpoint in the key clinical study, and will formally submit the marketing application of the drug to CDE in the near future.
Based on the excellent clinical efficacy, Hejilunsai Injection has been recognized as a "Breakthrough Therapy Drug" by the Drug Evaluation Center of the State Drug Administration and an orphan drug qualification by the US FDA. This product is the first in China to reach the end point of clinical research The CAR-T product in the field of leukemia is also expected to become the first domestically-independent innovative CAR-T product targeting the CD19 target.
4. Legend Biotech
On February 28, 2022 local time, Legend Biotech officially announced in Somerset, New Jersey, the United States that its self-developed cell therapy product Cedargiolund (English product name: CARVYKTI) was approved by the US FDA for marketing. For the treatment of patients with relapsed or refractory multiple myeloma (R/R MM) who have previously received four or more lines of therapy, including proteasome inhibitors, immunomodulators and anti-CD38 monoclonal antibodies. Since then, the drug has been approved for marketing in Europe and Japan in May and September this year, and its key Phase 2 clinical data for Chinese patients was published in November.
On November 21, 2022 local time, Legend Biotech officially announced in Somerset, New Jersey, USA that the US Food and Drug Administration (FDA) has approved the IND application for LB2102. LB2102 is an autologous chimeric antigen receptor T cell (CAR-T) therapy for the treatment of adult patients with extensive-stage small cell lung cancer (SCLC).
5. ImmunoChina
On October 31, 2022, ImmunoChina successfully completed the D+ round of strategic financing of hundreds of millions of yuan. This is another round of financing completed by ImmunoChina within one year after the D round of financing in November 2021 , the total financing amount during the year has exceeded RMB 500 million. The financing funds will be used to support the NDA application of IM19, the company's first CAR-T cell therapy product with independent intellectual property rights, to accelerate the process of commercialization and promote the drug's approval as soon as possible.
The FAI climbed 5.9 percent year-on-year in the first 11 months of 2018, quickening from the 5.7-percent growth in Jan-Oct, the National Bureau of Statistics (NBS) said Friday in an online statement.
The key indicator of investment, dubbed a major growth driver, hit the bottom in August and has since started to rebound steadily.
In the face of emerging economic challenges home and abroad, China has stepped up efforts to stabilize investment, in particular rolling out measures to motivate private investors and channel funds into infrastructure.
Friday's data showed private investment, accounting for more than 60 percent of the total FAI, expanded by a brisk 8.7 percent.
NBS spokesperson Mao Shengyong said funds into weak economic links registered rapid increases as investment in environmental protection and agriculture jumped 42 percent and 12.5 percent respectively, much faster than the average.
In breakdown, investment in high-tech and equipment manufacturing remained vigorous with 16.1-percent and 11.6-percent increases respectively in the first 11 months. Infrastructure investment gained 3.7 percent, staying flat. Investment in property development rose 9.7 percent, also unchanged.
 English
English

















































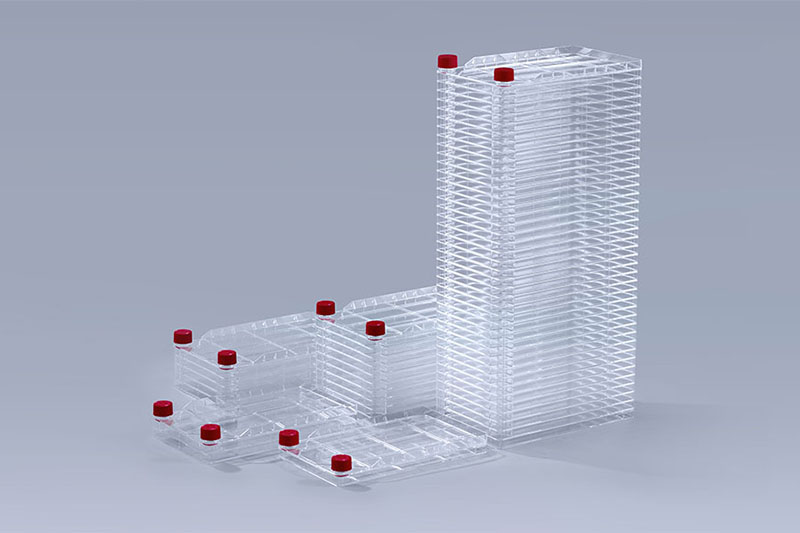 Cell Factory
Cell Factory Erlenmeyer Shake Flasks
Erlenmeyer Shake Flasks