May 10, 2023 the U.S. Food and Drug Administration (FDA) has approved Elfabrio (pegunigalsidase alfa-iwxj) in the United States for the treatment of adult patients with Fabry disease.
"While much progress has been made in the treatment of Fabry disease, there is still a need for new treatment options," said Giacomo Chiesi, head of Chiesi Global Rare Diseases. "We established Chiesi Global Rare Diseases to deliver innovative therapies and solutions for people affected by rare diseases. With the FDA approval of Elfabrio, we can now offer people living with Fabry disease an alternative treatment option."
"We are extremely pleased to receive FDA approval of Elfabrio for the treatment of adult patients with Fabry disease," said Dror Bashan, Protalix's President and Chief Executive Officer. "This approval is a testament to the dedication of the Protalix and Chiesi teams to deliver this much needed new therapeutic option to patients in need. The totality of clinical data suggests that Elfabrio has the potential to be a long-lasting therapy. Together with Chiesi, we are grateful to all of the patients and investigators and their staff members who participated in our clinical trial programs and remain committed to bringing Elfabrio to patients with Fabry disease."
Elfabrio is a PEGylated enzyme replacement therapy (ERT). It is a recombinant human α–Galactosidase–A enzyme expressed in plant-cell culture that is designed to provide a long half-life.
The safety, tolerability, and efficacy of Elfabrio has been studied in a comprehensive clinical development program in more than 140 patients with up to 7.5 years of follow up treatment. It has been studied in both ERT-naïve and ERT-experienced patients, including a head-to-head trial that met its primary endpoint with Elfabrio demonstrating non-inferior efficacy to agalsidase beta in controlling estimated glomerular filtration rate (eGFR) decline, and in which Elfabrio was generally well-tolerated with the majority of adverse events being mild or moderate in severity.
"It is important to understand that there is a lot of variability in Fabry disease and misdiagnoses are common, especially in women," said Jack Johnson, founder of the Fabry Support & Information Group (FSIG). "Growing up, a lot of people didn't know what was wrong with me. They knew I was different, but they didn't know why. Now we have made advances in screening, treatment, and monitoring for Fabry disease."
*Elfabrio has an initial half-life of 78.9 ± 10.3 hours. Clinical studies have not established that half-life results in superior efficacy or safety based on clinically relevant end points.
Source: https://www.drugs.com/newdrugs/fda-approves-elfabrio-pegunigalsidase-alfa-iwxj-adult-patients-fabry-6012.html
The FAI climbed 5.9 percent year-on-year in the first 11 months of 2018, quickening from the 5.7-percent growth in Jan-Oct, the National Bureau of Statistics (NBS) said Friday in an online statement.
The key indicator of investment, dubbed a major growth driver, hit the bottom in August and has since started to rebound steadily.
In the face of emerging economic challenges home and abroad, China has stepped up efforts to stabilize investment, in particular rolling out measures to motivate private investors and channel funds into infrastructure.
Friday's data showed private investment, accounting for more than 60 percent of the total FAI, expanded by a brisk 8.7 percent.
NBS spokesperson Mao Shengyong said funds into weak economic links registered rapid increases as investment in environmental protection and agriculture jumped 42 percent and 12.5 percent respectively, much faster than the average.
In breakdown, investment in high-tech and equipment manufacturing remained vigorous with 16.1-percent and 11.6-percent increases respectively in the first 11 months. Infrastructure investment gained 3.7 percent, staying flat. Investment in property development rose 9.7 percent, also unchanged.
 English
English

















































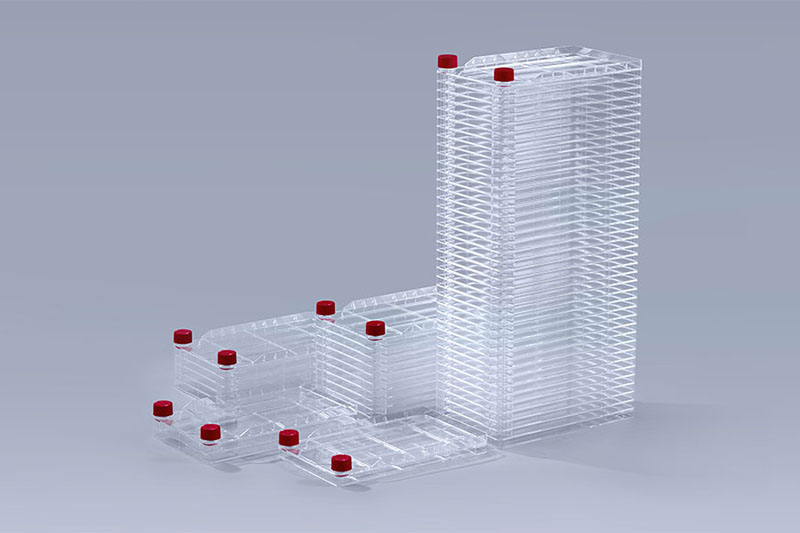 Cell Factory
Cell Factory PETG Media Bottles
PETG Media Bottles